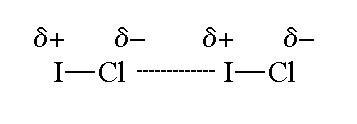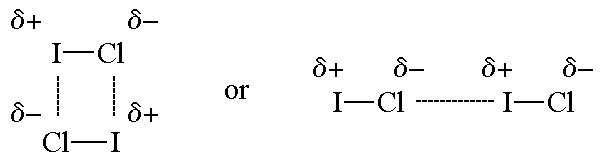A comparative view on the potential acting on an electron in a molecule and the electrostatic potential through the typical halogen bonds - Bartashevich - 2018 - Journal of Computational Chemistry - Wiley Online Library
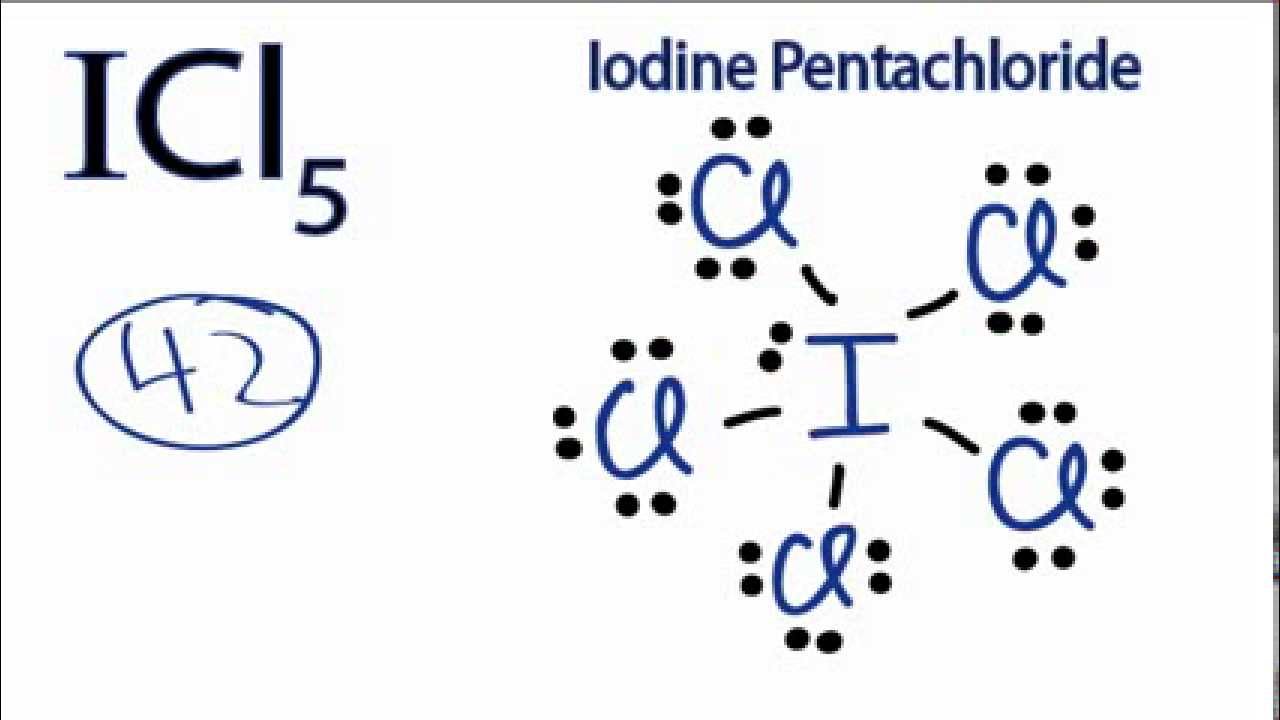
![ICl 4 – 36 e – EDG: octahedral MG: sq. planar [ ].. I –Cl Cl–.. Cl.. – For molecules with more than one central atom, simply apply the VSEPR model to each. - ppt download ICl 4 – 36 e – EDG: octahedral MG: sq. planar [ ].. I –Cl Cl–.. Cl.. – For molecules with more than one central atom, simply apply the VSEPR model to each. - ppt download](https://images.slideplayer.com/34/10194070/slides/slide_3.jpg)
ICl 4 – 36 e – EDG: octahedral MG: sq. planar [ ].. I –Cl Cl–.. Cl.. – For molecules with more than one central atom, simply apply the VSEPR model to each. - ppt download
![ICl 4 – 36 e – EDG: octahedral MG: sq. planar [ ].. I –Cl Cl–.. Cl.. – For molecules with more than one central atom, simply apply the VSEPR model to each. - ppt download ICl 4 – 36 e – EDG: octahedral MG: sq. planar [ ].. I –Cl Cl–.. Cl.. – For molecules with more than one central atom, simply apply the VSEPR model to each. - ppt download](https://slideplayer.com/10194070/34/images/slide_1.jpg)
ICl 4 – 36 e – EDG: octahedral MG: sq. planar [ ].. I –Cl Cl–.. Cl.. – For molecules with more than one central atom, simply apply the VSEPR model to each. - ppt download