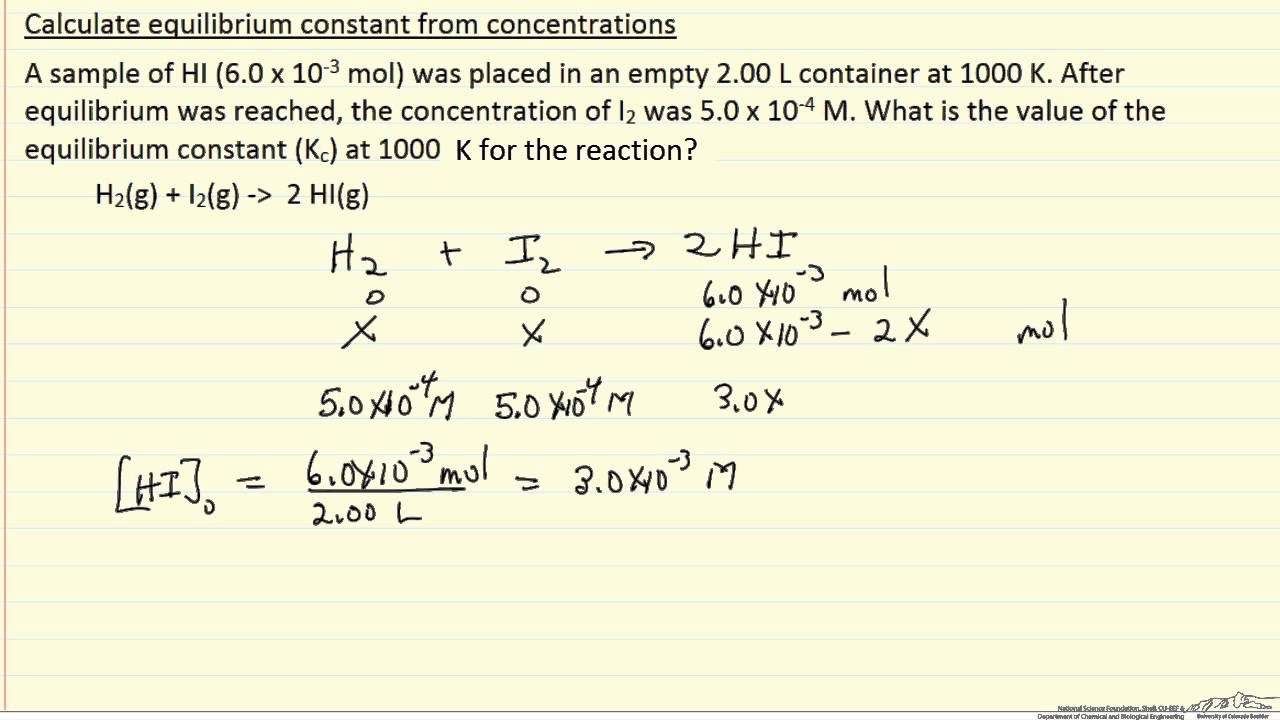
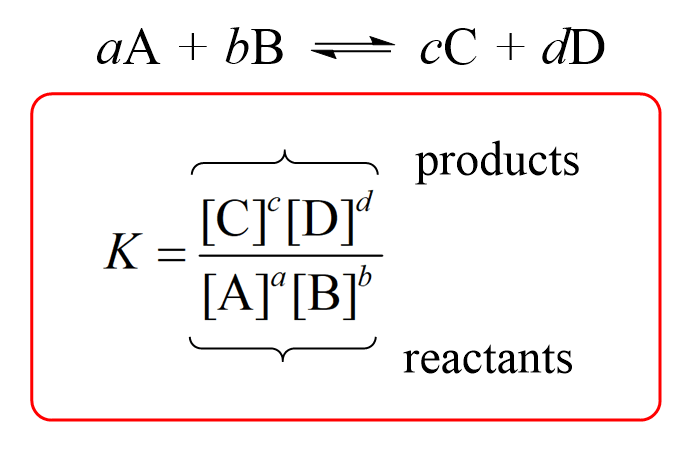
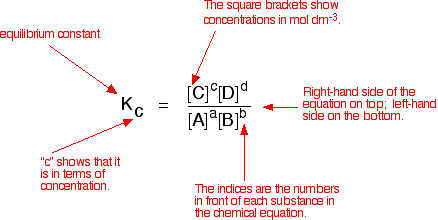
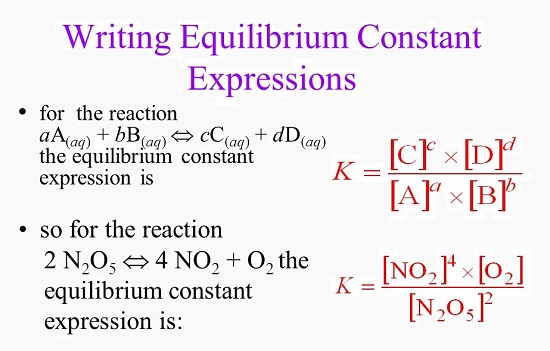
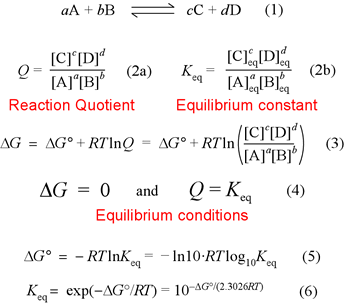
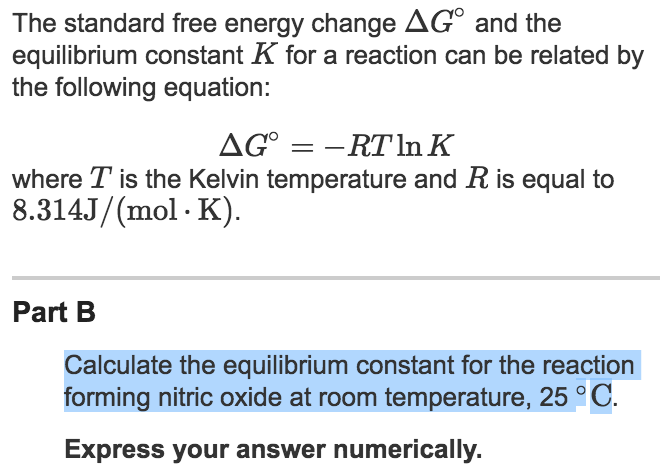
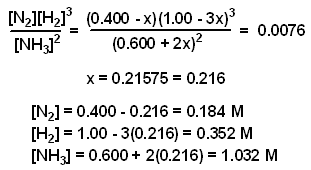7.2 The Position of Equilibrium.. Assessment Statements Deduce the equilibrium constant expression (K c ) from the equation for a homogeneous reaction. - ppt download
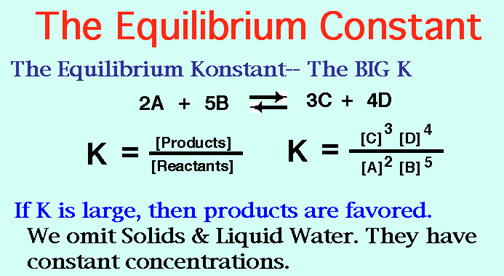
How does the value of an equilibrium constant relate to the relative quantities of reactants and products at equilibrium? | Socratic

Question Video: Calculating the Equilibrium Constant for Partial Pressures Given the Partial Pressure of Each Species | Nagwa

Calculate the equilibrium constant for the reaction at 298K. `Zn(s) +Cu^(2+)(aq) hArr Zn^(2+)(aq) +C - YouTube
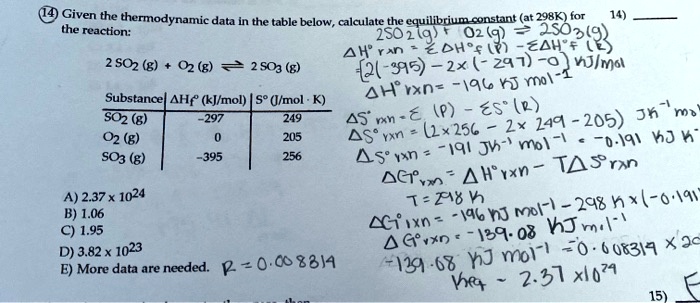
SOLVED: Given - the thermodynamic data in the table below, calculate the equilibrium constant (at 298K) for the reaction: 250 2 02 2502 9 3 Z4H" = 8 4h" in Dh"f SO2 (

Calculate the equation constant for the reaction: H2 (g) + CO2 (g) H2O (g) + CO at 1395 K, if the equilibrium constants at 1395 K for the following are 2H2O (g)
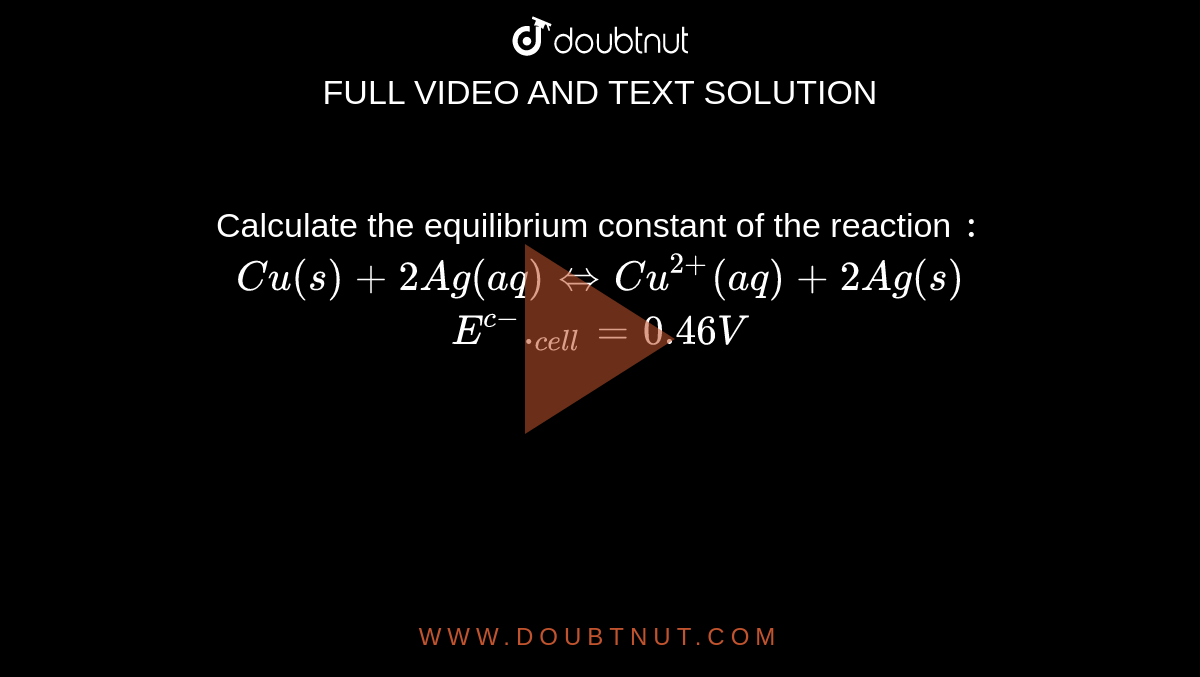
Calculate the equilibrium constant of the reaction : Cu(s)+2Ag(aq) hArrCu^(2+)(aq) +2Ag(s) E^(c-).(cell)=0.46V