![PDF] Evaluation of the Discrepancy between the European Pharmacopoeia Test and an Adopted United States Pharmacopoeia Test Regarding the Weight Uniformity of Scored Tablet Halves: Is Harmonization Required? | Semantic Scholar PDF] Evaluation of the Discrepancy between the European Pharmacopoeia Test and an Adopted United States Pharmacopoeia Test Regarding the Weight Uniformity of Scored Tablet Halves: Is Harmonization Required? | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/0219ae49f3ffe1a993893eabe666b5a77ba0a4e2/5-TableII-1.png)
PDF] Evaluation of the Discrepancy between the European Pharmacopoeia Test and an Adopted United States Pharmacopoeia Test Regarding the Weight Uniformity of Scored Tablet Halves: Is Harmonization Required? | Semantic Scholar
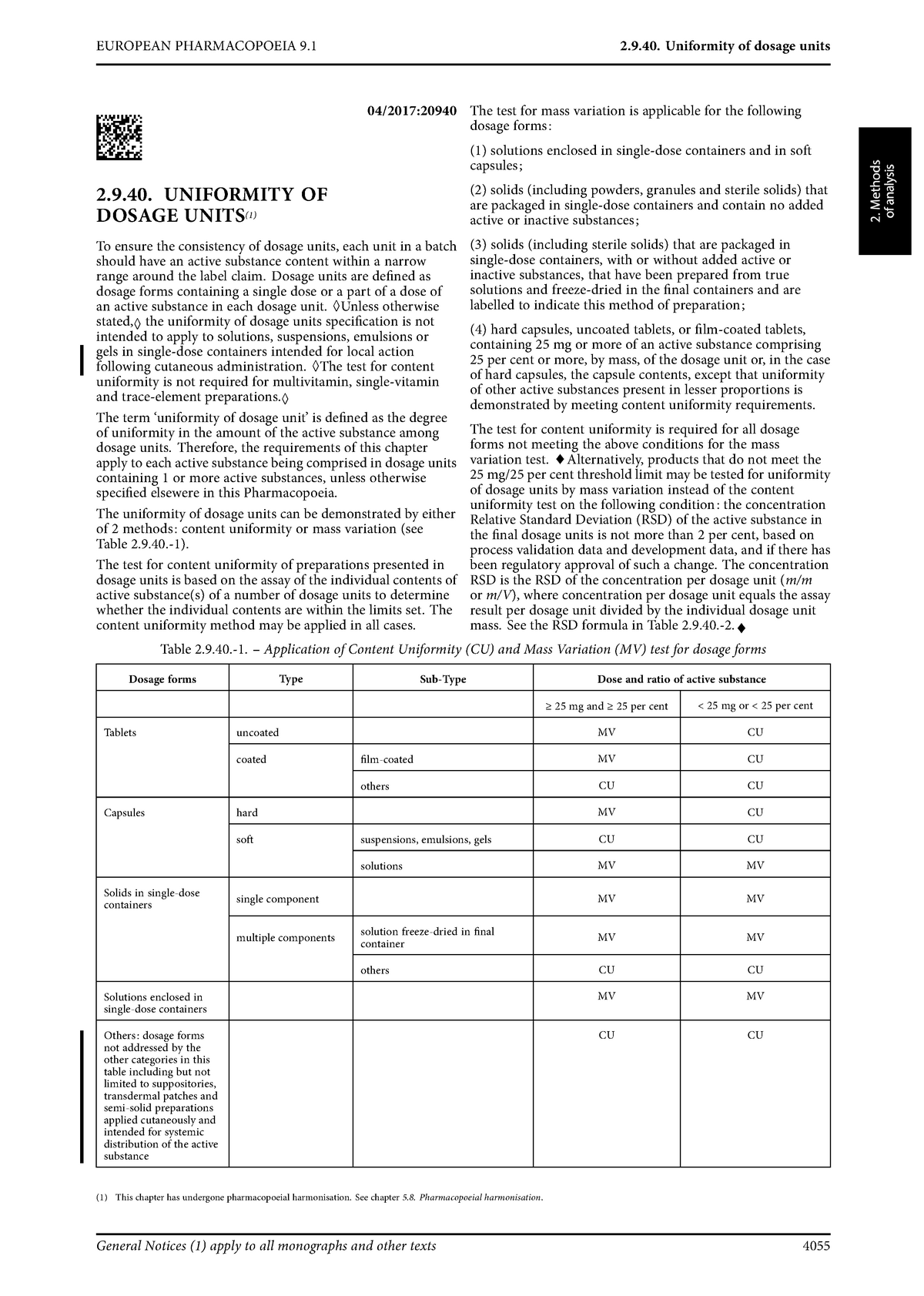
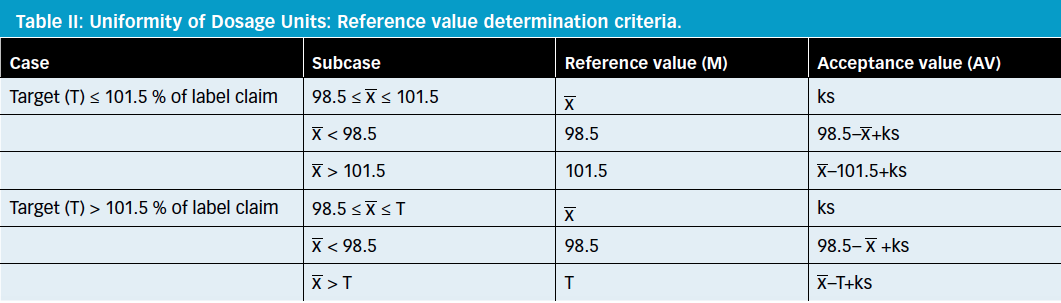
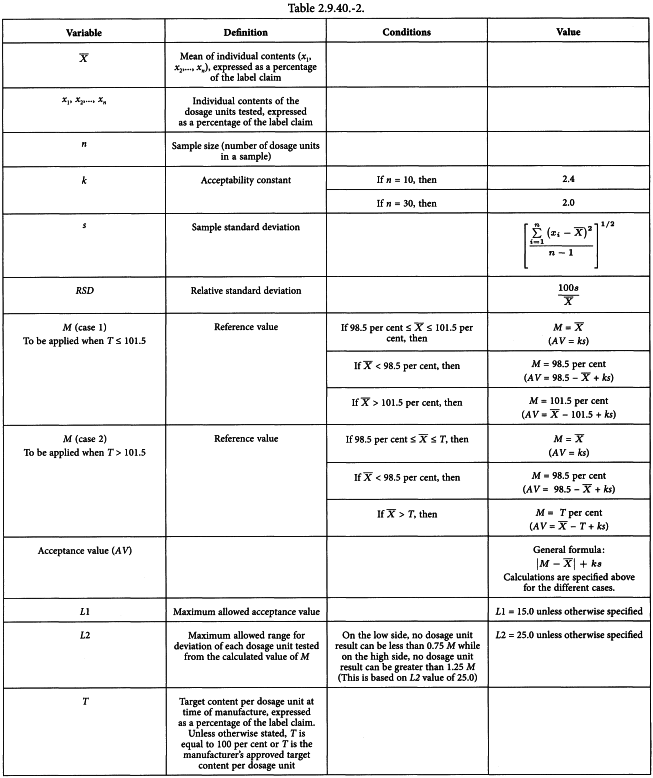
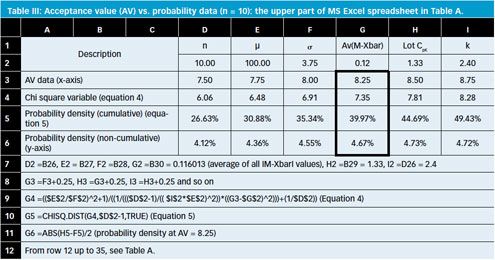
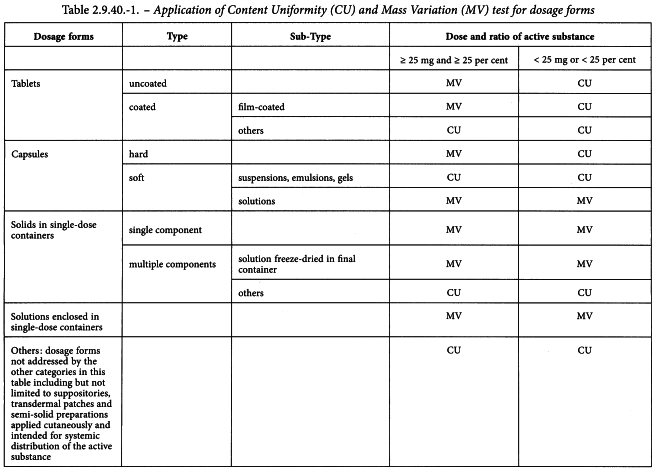
Uniformity of dosage units - EUROPEAN PHARMACOPOEIA 9 2.9. Uniformity of dosage units 04/2017: 2.9. - Studocu
Semi-solid rectal preparations Rectal foams Rectal tampons SEMI-SOLID PREPARATIONS FOR CUTANEOUS APPLICATION Praeparationes moll

EUROPEAN PHARMACOPOEIA & PHARMACOPOEIA INTERNATIONALS (INTERNATIONAL PHARMACOPOEIA) | The Pharmapedia

Uniformity of dosage units—comparative study of methods and specifications between Eur. Pharm. 3rd and USP 23 - ScienceDirect

Weight and content uniformity of lorazepam half-tablets: A study of correlation of a low drug content product – topic of research paper in Chemical sciences. Download scholarly article PDF and read for
Q4B Evaluation and Recommendation of Pharmacopoeial Texts for Use in the ICH Regions Annex 6 Uniformity of Dosage Units General Chapter
![PDF] Weight and content uniformity of lorazepam half-tablets: A study of correlation of a low drug content product. | Semantic Scholar PDF] Weight and content uniformity of lorazepam half-tablets: A study of correlation of a low drug content product. | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/3f129f060436923435cbb5e514e797c1b6799cb1/3-Table2-1.png)
PDF] Weight and content uniformity of lorazepam half-tablets: A study of correlation of a low drug content product. | Semantic Scholar